The Developmental and Reproductive Toxicant Identification Committeevoted unanimously to list bisphenol S as a reproductive toxicant under Prop 65
On Dec. 29, the California Office of Environmental Health and Hazard Assessment (OEHHA) formally added bisphenol S (BPS) as a reproductive toxicant under the Safe Drinking Water and Toxic Enforcement Act of 1986, commonly known as Prop 65. The decision to list BPS under Prop 65 will affect manufacturers across a variety of industries, as BPS can be found in an array of consumer products, including sportswear, raingear, infant clothing, blankets, curtains, food can coatings, nonstick pans, and thermally printed receipts produced at gas pumps and cash registers.
BPS and other bisphenols are common replacements to bisphenol A (BPA), which was listed under Prop 65 as a reproductive toxicant in 2015 and as adevelopmental toxicant in 2020. Products resulting in significant exposure to BPS will require warnings starting one year from the listing date, Dec. 29, 2024.
Weighing the evidence for Prop 65 listing of bisphenol S
The listing follows a virtual meeting on Dec. 12 where OEHHA convened the Developmental and Reproductive Toxicant Identification Committee (DARTIC), which "serves as the state's qualified experts for determining whether a chemical has been clearly shown to cause reproductive toxicity."
During the Dec. 12 meeting and vote, participants referenced a 137-page hazard identification document, "Evidence on the Female Reproductive Toxicity of Bisphenol S," published in October 2023 by OEHHA. The document identifies 23 human epidemiologic studies of possible effects of BPS on the female reproductive system. These were cohort, case-control, or cross-sectional studies that assessed individual-level exposure to BPS and several different female reproductive outcomes, including gestational diabetes, thyroid hormones, sex steroid hormones, and polycystic ovary syndrome (PCOS).
The document also includes a review of 43 animal studies and related pharmacokinetics of BPS. According to the pharmacokinetics section of the document, BPS has been found in human blood, umbilical cord blood, amniotic fluid, breast milk, semen, skin, and urine but has not been found to accumulate in tissues or blood over time.
In the Dec. 12 meeting, DARTIC voted 9 to 0 to list BPS as a female reproductive toxicant, stating that BPS "was clearly shown through scientifically valid testing according to generally accepted principles to cause female reproductive toxicity."
Potential future regulatory developments for bisphenol S
DARTIC will convene again in 2024 to consider the effects of BPS on male reproductive toxicity and developmental toxicity. Other countries are also considering legislation concerning BPS. In Jan. 2023, the European Chemicals Agency (ECHA) added BPS to the candidate list for substance of very high concern (SVHC) designation while it investigates BPS for reproductive toxicity and endocrine disruption. ECHA also lists Bisphenol A, Bisphenol B, and 2,2-bis(4'-hydroxyphenyl)-4-methylpentane as SVHCs.
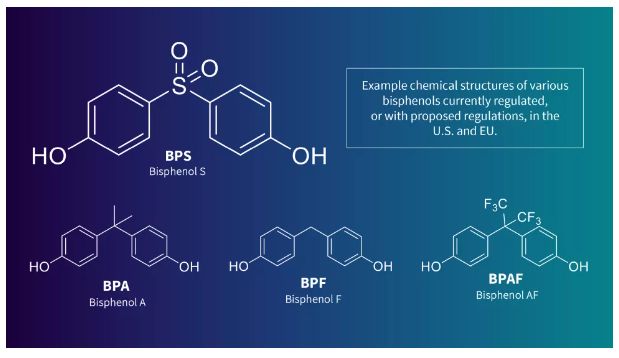
Related substances are also being monitored for potential restrictions, making the search for viable alternatives even more challenging. In 2022, Germany proposed additional restrictions on Bisphenol F and Bisphenol AF and its salts, as they meet the World Health Organization criteria for endocrine disruptors in the environment. Other ECHA Member States have recommended the restriction of more than 30 bisphenols due to their potential hormonal or reprotoxic effects.
Originally published 10 January 2024
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.





