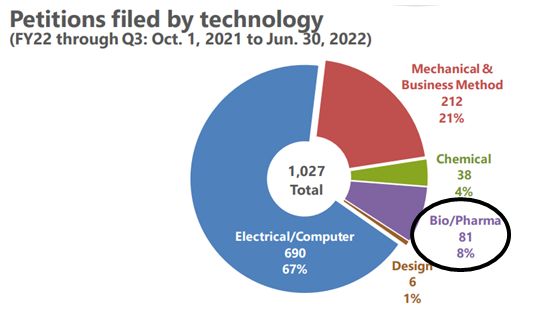
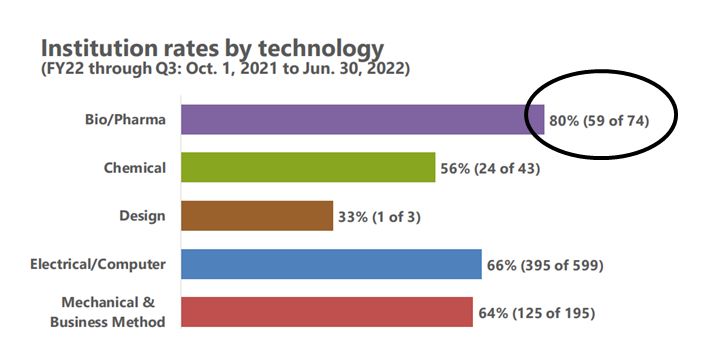
When it comes to IPR petitions filed in the Bio/Pharma space, USPTO data tells us that while Bio/Pharma petitions make up only 8% of the total petitions filed for the fiscal year of 2022 (through June 30, 2022), there is a high intuition rate for those Bio/Pharma petitions. According to USPTO statistics, 80% of the Bio/Pharma cases have been instituted so far this year. Put differently, 59 of the 74 petitions reviewed were subsequently granted.
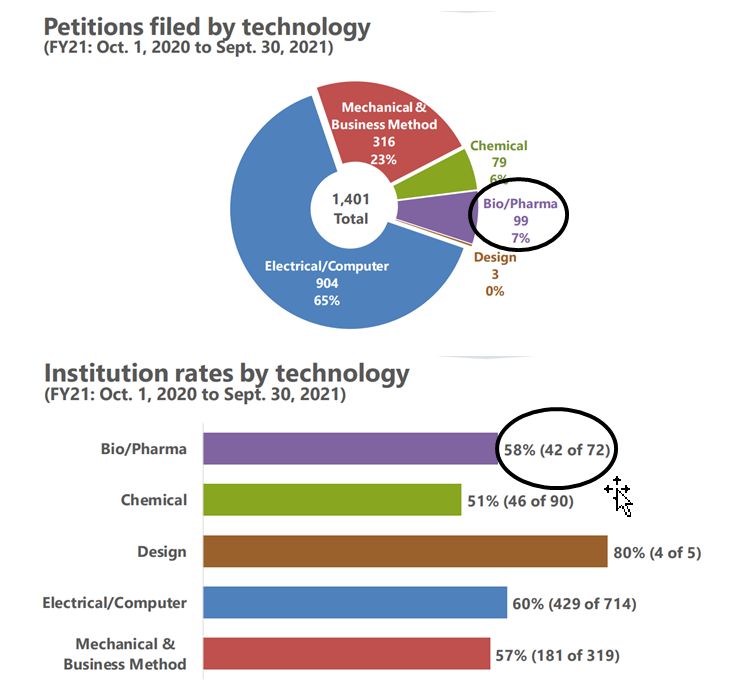
In 2021, there were 99 total Bio/Pharma IPR petitions filed, but a lower percentage of granted cases compared to 2022. Of the 72 petitions reviewed, 42 were subsequently granted, resulting in a 52% institution rate in 2021. So while there are less overall Bio/Pharma filings in 2022 (so far), the institution rate is considerably higher.
In terms of Bio/Pharma IPRs filed overall, the numbers have not seen a big change over the past few years. The overall trend shows there has been a steady decline in petition filings since 2017. See our previous post on Bio/Pharma IPRs here. But while the overall numbers have not varied much, there are reasons to for us to believe that Bio/Pharma patents could be facing closer review in the future.
On July 28, 2022, Director Kathi Vidal published a blog post where she emphasized the initiatives being taken by the USPTO, one of which relates to the duty of disclosure and duty of reasonable inquiry. Director Vidal explained:
"This duty applies during examination of patent applications, including continuation applications, and after issuance during any post-grant examination or proceeding to review the issued patent. Adherence to these duties helps our patent examiners and administrative patent judges within the Patent Trial and Appeal Board effectively and efficiently determine whether an invention—for instance, a drug product—is patentable by providing them with key relevant information. Failure to abide by these duties is not only a disservice to the American public, it is sanctionable."
The full blog post can be found here. To reinforce these duties, the USPTO, in a federal register notice published on July 29, 2022, announced more information on the duties of disclosure and reasonable inquiry. The federal register notice ("FRN") focuses on the duty of candor and good faith in dealing with the USPTO to disclose information material to the patentability of the claimed invention. Specifically, the FRN states that each party that submits a paper to the USPTO has "an additional duty to perform an inquiry that is reasonable under the circumstances, including reviewing documents to identify information that is material to the patentability of a claimed invention." The FRN further states, "in the pharmaceutical space, the duties promote robust and reliable patents that incentivize and protect innovation that brings life-saving drugs to the American people while not unnecessarily delaying more affordable generic drugs." The full federal register notice can be found here.
In addition, Director Vidal noted that the USPTO is also considering additional measures to "ensure issued patents are robust and reliable." These additional measures broadly include:
- Enhancing collaboration with other agencies, such as the FDA, on key technology areas, including pharmaceuticals and biologics
- Improving procedures for obtaining a patent to ensure that the USPTO issues robust and reliable patents
- Improving the process for challenging issued patents before the Patent Trial and Appeal Board (America Invents Act proceedings)
- Improving public participation in the patent system
- Considering new proposals for incentivizing and protecting innovation while minimizing unnecessary delays in getting more affordable drugs to market
More information on these measures and the USPTO's drug pricing initiatives can be found here.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


